
Het aantal neutronen, protonen en elektronen bepalen wikiHow
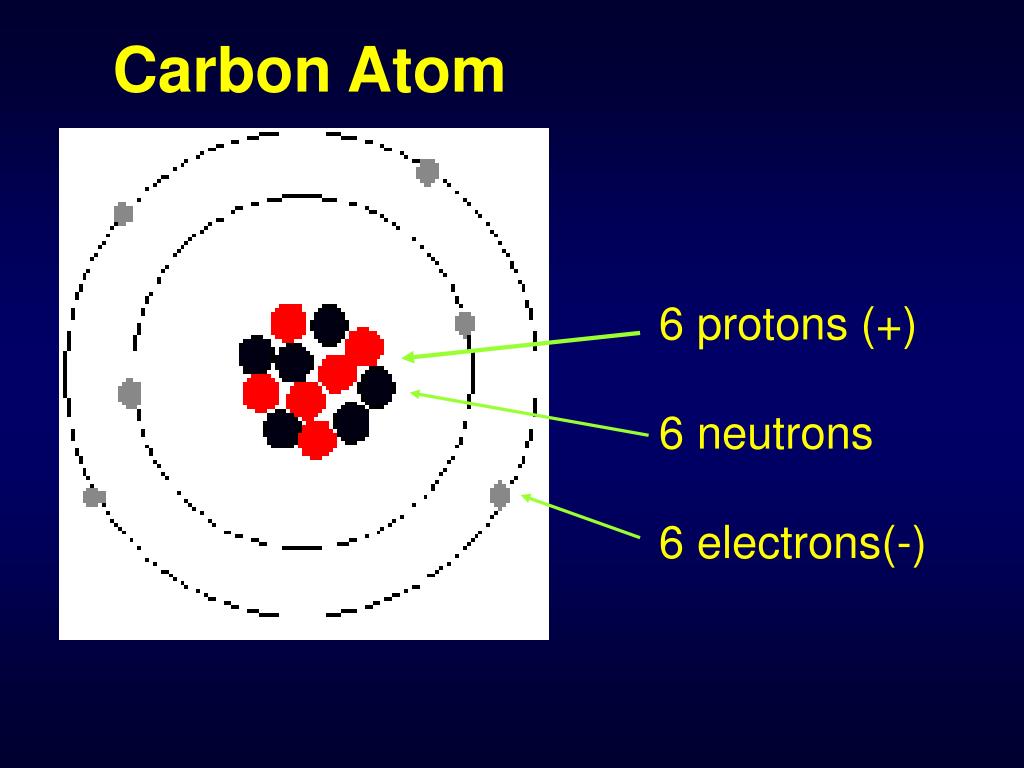
Carbon is a chemical element. Its atomic number is 6; its atomic weight is 12.011. It is a group IVA element, residing between boron and nitrogen on the periodic table, and it has 6 protons, 6 neutrons, and 6 electrons.

Human soul image by Monica Mitchell on (ಠ_ರೃ) Knowlédgé Protons, Electrons
Study with Quizlet and memorize flashcards containing terms like What is the atomic number of an atom that has 6 protons, 6 neutrons, and 6 electrons? 12 0 18 -1 6, Which of these refers to atoms with the same atomic number but different atomic masses? These atoms have different numbers of protons. These atoms have different numbers of electrons. These atoms are isomers. These atoms are.
PPT Introduction to Mass Spectrometry PowerPoint Presentation, free download ID1273270
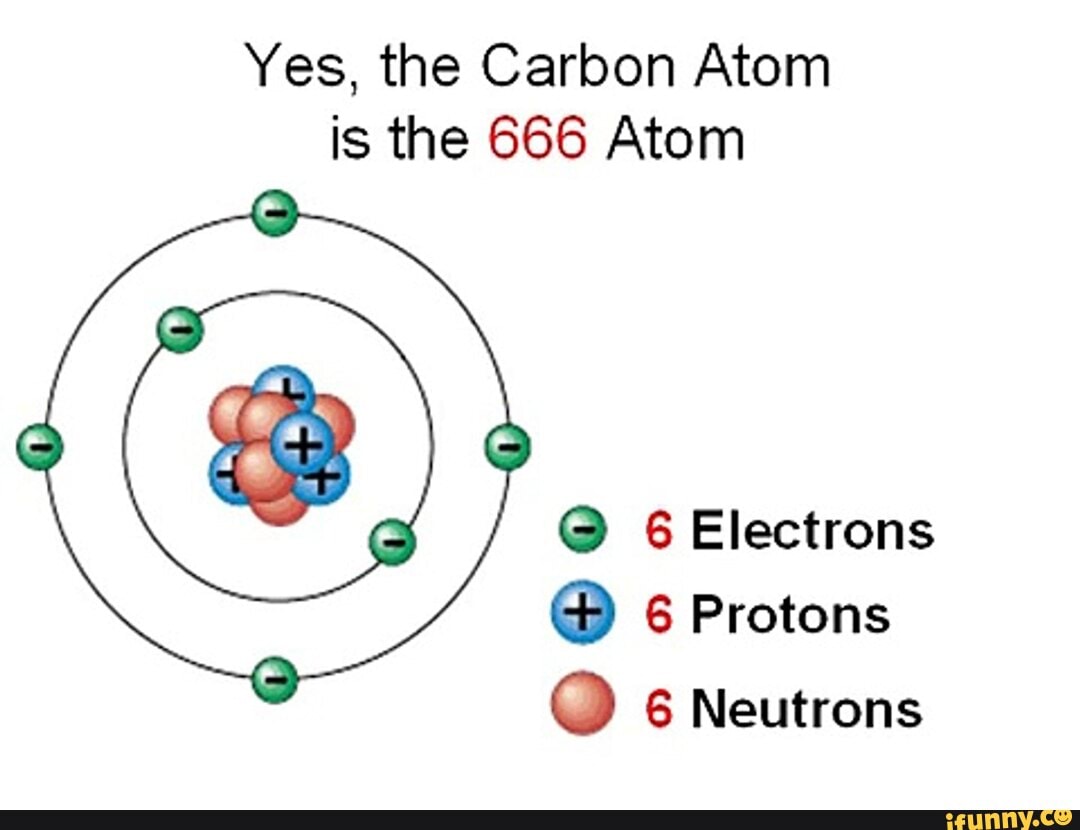
That number is 666. Some people are really very scared of this Biblical passage because of the number 666…we all know why…. However, this has another meaning altogether - it is referring to the subatomic particles that make up carbon: 6 protons, 6 neutrons, 6 electrons. Carbon's atomic number is 6 and it is the 6th most bountiful.

Nucleons, Atomic Number and Mass Number Definitions.. and more
Resources. Education. Graphite 101. Carbon by the Number. Carbon is a chemical element. Its atomic number is 6; its atomic weight is 12.011. It is a group IVA element, residing between boron and nitrogen on the periodic table, and it has 6 protons, 6 neutrons, and 6 electrons. The electron configuration is 1s2 , 2s2, 2p2.
Periodic Table Of Elements List With Protons Neutrons And Electrons Awesome Home
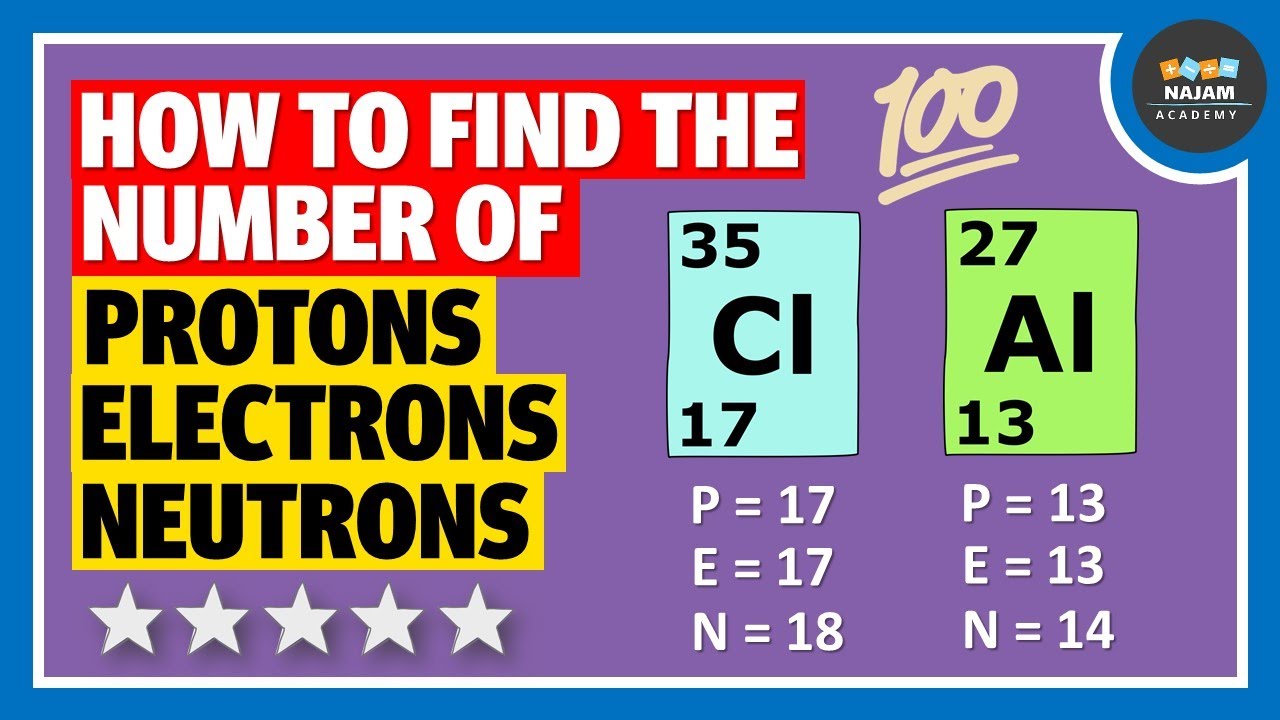
Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 - (1-) = 54]. Exercise. An ion of platinum has a mass number of 195 and contains 74 electrons.
How to find the number of Protons, Neutrons and Electrons? Chemistry YouTube
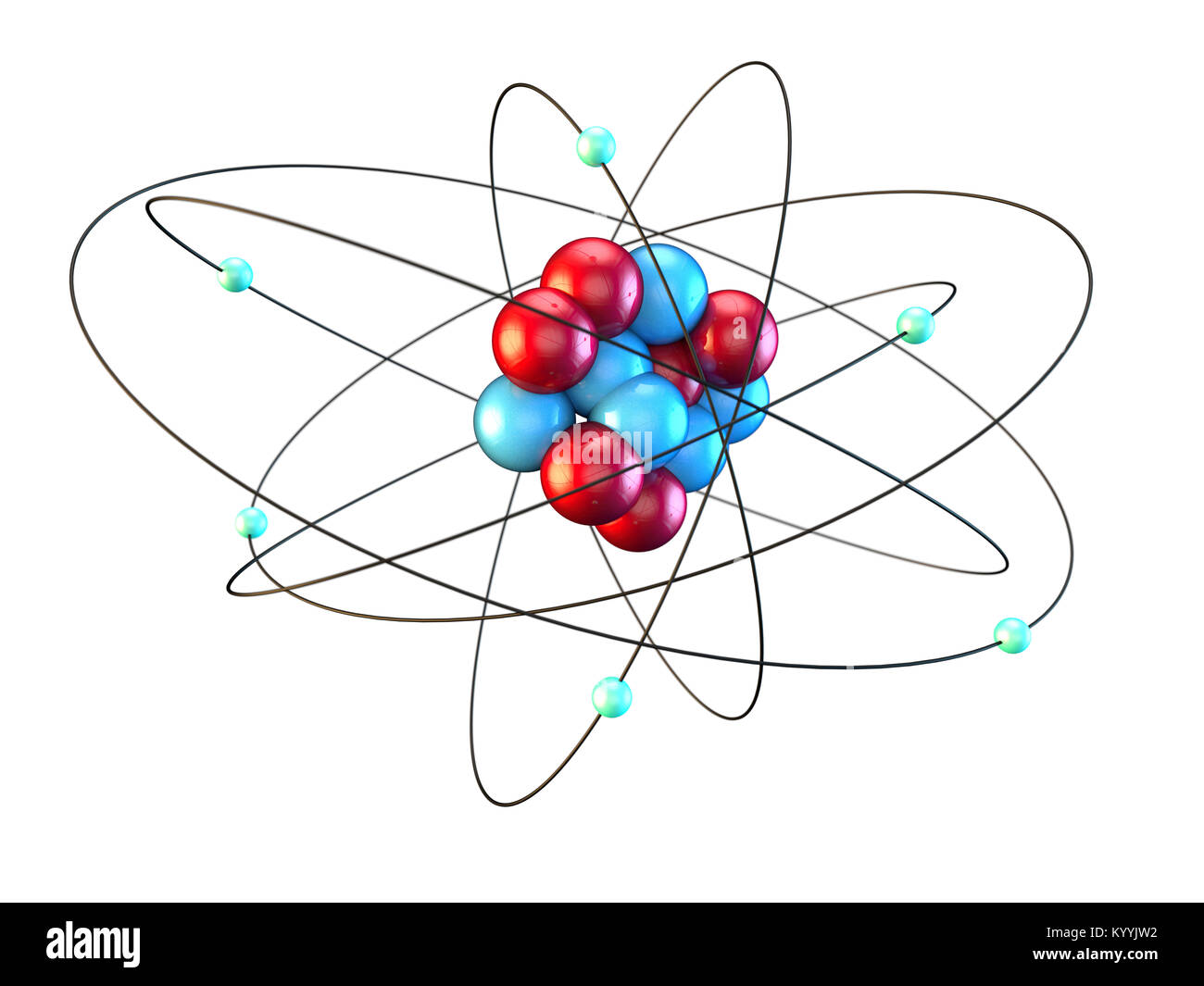
It has an atomic number of 6. That means a carbon atom has 6 protons, 6 neutrons, and 6 electrons. Since carbon is in the second row (or second period), it has 2 electron orbits. Use the clay to make your protons and neutrons in the nucleus. The nucleus should be about the size of a large gumball. (Use two different colors of clay.)
Periodic Table Of Elements List With Protons Neutrons And Electrons Awesome Home
Protons and Neutrons in Carbon. Carbon is a chemical element with atomic number 6 which means there are 6 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
.PNG)
Periodic Table Of Elements Number Of Protons Neutrons And Electrons About Elements
So yeah, " carbon " has six protons, six neutrons and six electrons. Carbon-12 specifically, which accounts for 98.9 percent of all carbon. The chemical formula for melanin is C 18 H 10 N 2 O 4. That means there are 18 carbon atoms, 10 hydrogen, 2 nitrogen and 4 oxygen atoms in a melanin molecule. Melanin is carbon, hydrogen, nitrogen and.

Protons neutrons and subatomic particles Pharmacy Gyan
The number of protons plus the number of neutrons equals the atomic mass of the element based upon atomic mass units (amus) For this element 6 protons and 6 neutrons combine to make an atomic mass of 12 amus. Lastly, the values of protons and electrons tell whether the atom is an ion or neutral. When protons equal electrons the atom is neutral.
Carbon atom showing six electrons orbiting six protons and six neutrons, chemistry chemical
Study with Quizlet and memorize flashcards containing terms like What is the atomic number of an atom that has 6 protons, 6 neutrons, and 6 electrons? 12 6 18 0 -1, Which of these refers to atoms with the same atomic number but different atomic masses? These atoms have different numbers of protons. These atoms have different numbers of electrons. These atoms are different elements. These atoms.
Yes, the Carbon Atom is the 666 Atom 6 Electrons 6 Protons 6 Neutrons iFunny
Flexi Says: Carbon-12 is composed of 6 protons, 6 neutrons, and 6 electrons.

Periodic Table Electrons And Protons Periodic Table Timeline 1EE
Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number. A property closely related to an atom's mass number is its atomic mass.

Electrons — Structure & Properties Expii
Each atom has a charged sub-structure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons. The number of protons and the mass number of an atom define the type of atom. Atoms of the same element with different mass numbers are called isotopes. Created by Jay.

666 = The Beast/ Carbon Body 6 Protons, 6 Neutrons, 6 Electrons Spirit science, Physics and
Study with Quizlet and memorize flashcards containing terms like An atom has 6 protons, 8 neutrons, and 6 electrons. Its atomic mass is:, An atom with 6 protons, 7 neutrons, and 6 electrons shares four pairs of electrons with four other atoms. This atom is now considered to be :, Nucleotides are composed of and more.

Periodic Table Carbon Protons Neutrons Electrons Periodic Table Timeline
Explanation: atomic number =number of protons. Therefore it is 6. Answer link. The atomic number is 6, for carbon. The atomic number is equal to the number of protons.

Atoms & Molecules echapter — The Biology Primer
It is, rather, the number of protons in the nucleus, which we call the atomic number and denote by the symbol Z. Each proton carries an electric charge of +1, so the atomic number also specifies the electric charge of the nucleus. In the neutral atom, the Z protons within the nucleus are balanced by Z electrons outside it.